Other Brands/Names
This medicine is marketed under the brand name Nesp.
How It Works
Darbepoetin contains a synthetic form of erythropoietin, a hormone that stimulates red blood cell (erythrocyte) production in the bone marrow, leading to the formation of mature red blood cells. In anemia caused by insufficient red blood cells, this effect is expected to increase the circulating red blood cell count.
Indications
This drug is used in anemic patients associated with chronic kidney disease or patients receiving chemotherapy for cancer.
Contraindication
There are several medical conditions contraindicared for this drug, namely:
-
Previous allergic reaction to this medicine.
-
Uncontrolled hypertension.
Side Effects
The following are the side effects of this drug:
-
High or low blood pressure.
-
Chest pain.
-
Fatigue.
-
Fever.
-
Headache or dizziness.
-
Generalized body pain.
-
Increased potassium levels.
-
Shortness of breath.
-
Cough.
-
Transient increase in platelet counts.
-
Edema.
-
Joint pain.
-
Infection.
-
Skin rash.
Types
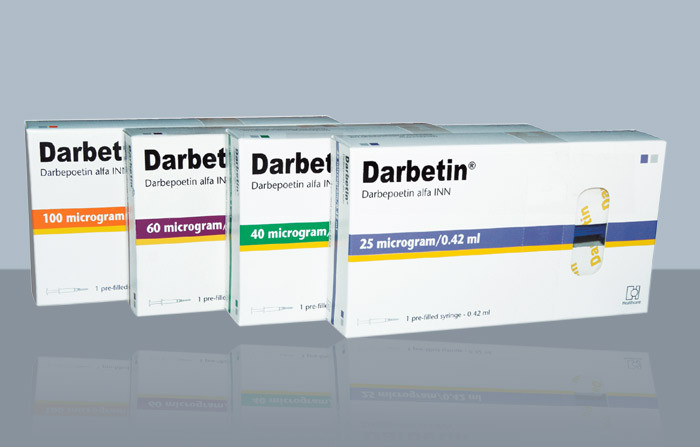
This drug is available as 30 mcg/mL, 40 mcg/mL, 60 mcg/mL, and 100 mcg/mL injection medications.
Dosage
Drug doses vary according to the purpose of treatment, but patients will receive injections. The following is a dose of darbepoetin:
Anemia in chronic kidney disease
-
Adults: initial dose is 0.45 mcg/kg once weekly, or 0.75 mcg/kg every 2 weeks, or 1.5 mcg/kg once monthly.
-
Adjust the dose by about 25% of the initial dose every 4 weeks as needed based on hemoglobin levels.
-
Usual recommended dosage is 0.45 mcg/kg every 1–2 weeks, with dose increases permitted to reach the target hemoglobin.
-
Pediatric dosage follows the same weight-based approach as adults.
Anemia in cancer patients with chemotherapy
-
Initial dose is 500 mcg every 3 weeks, or 2.25 mcg/kg once weekly.
-
Titrate the dose according to hemoglobin response. Treatment may be stopped after 4 weeks and at the end of chemotherapy.
Safety
-
Pregnancy Category C: animal studies show fetal risk, and adequate human data are lacking.
-
It is unknown whether the drug is excreted in breast milk.
Drug Interactions
This drug may interact with antihypertensives such as captopril and lisinopril, with possible decreases in blood pressure and increases in serum potassium.
Looking for more information about other drugs? Click here!
- dr Hanifa Rahma
Darbepoetin Alfa – Mims Indonesia. (2022). Retrieved 16 July 2022, from https://www.mims.com/malaysia/drug/info/darbepoetin%20alfa?mtype=generic
Darbepoetin Alfa injection – MedlinePlus. (2022). Retrieved 16 July 2022, from https://medlineplus.gov/druginfo/meds/a604022.html
Darbepoetin Alfa (Injection Route) - Mayo Clinic. Mayoclinic.org. (2021). Retrieved 16 July 2022, from https://www.mayoclinic.org/drugs-supplements/darbepoetin-alfa-injection-route/side-effects/drg-20068072?p=1
Darbepoetin Alfa -Uses, Side Effects and More – WebMD. (2022). Retrieved 16 July 2022, fromhttps://www.webmd.com/drugs/2/drug-21885/darbepoetin-alfa-albumin-injection/details